Limiting and excess reactants pogil delve into the captivating world of chemical reactions, revealing the intricate interplay between reactants and products. By exploring the concepts, applications, and experimental determination of limiting and excess reactants, this guide empowers students with a profound understanding of stoichiometry and its practical implications.
Stoichiometry provides a roadmap for predicting the course of a chemical reaction, allowing us to determine the limiting reactant—the reactant that is entirely consumed, dictating the maximum amount of product that can be formed. Identifying the excess reactant, on the other hand, unveils the reactant that remains unconsumed, providing valuable insights into reaction efficiency and potential side reactions.
Limiting and Excess Reactants
In a chemical reaction, the reactants are the initial substances that undergo transformation to form new substances known as products. The concept of limiting and excess reactants is crucial in understanding the stoichiometry of a reaction and predicting the outcome.
Definition
Limiting reactant is the reactant that is completely consumed in a chemical reaction, thereby limiting the amount of product that can be formed. On the other hand, excess reactant is the reactant that remains in excess after the reaction is complete.
Significance
Identifying limiting and excess reactants is significant because it allows us to:
- Determine the maximum amount of product that can be formed.
- Calculate the theoretical yield of the reaction.
- Predict the composition of the reaction mixture at equilibrium.
Stoichiometry and Limiting Reactants
Stoichiometry plays a crucial role in determining the limiting reactant in a chemical reaction. It involves the analysis of the stoichiometric ratios between reactants and products, which are derived from the balanced chemical equation.
Calculating the Limiting Reactant
To calculate the limiting reactant, stoichiometric ratios are used to compare the relative amounts of reactants available in the reaction. The reactant that is present in the smallest relative amount, relative to the stoichiometric ratio, is the limiting reactant.
For example, consider the following balanced chemical equation:
2H2 + O2 → 2H2O
If we have 4 moles of H2 and 2 moles of O2, we can compare the mole ratios to the stoichiometric ratio:
Mole ratio of H2: 4 moles / 2 = 2
Mole ratio of O2: 2 moles / 1 = 2
Since both mole ratios are equal, neither reactant is in excess. Therefore, both H2 and O2 are present in the stoichiometric ratio and are fully consumed in the reaction.
Identifying Excess Reactants
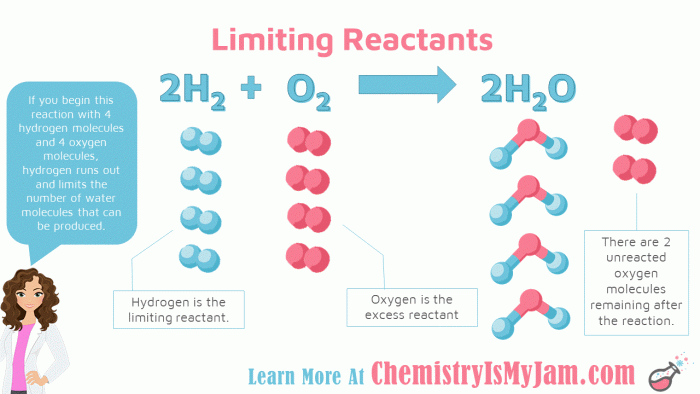
Identifying the excess reactant in a reaction is crucial for determining the limiting reactant and predicting the reaction’s outcome. There are several methods to identify the excess reactant:
- Compare the mole ratios:Calculate the mole ratio of the reactants based on their stoichiometric coefficients. The reactant with a higher mole ratio compared to the other reactant is likely to be in excess.
- Check the reaction progress:Monitor the reaction over time and observe which reactant is consumed first. The reactant that remains after the reaction is complete is the excess reactant.
- Analyze the equilibrium constant:For reversible reactions, the equilibrium constant can indicate the relative amounts of reactants and products at equilibrium. If the equilibrium constant is large, it suggests that the reaction favors the formation of products, implying that one of the reactants is in excess.
Implications of Excess Reactants
Having an excess reactant in a reaction has several implications:
- Increased reaction yield:The excess reactant ensures that the limiting reactant is completely consumed, leading to a higher yield of the desired product.
- Prevention of side reactions:Excess reactants can suppress unwanted side reactions by consuming the limiting reactant before it can react with other species.
- Control of reaction rate:In some cases, an excess of one reactant can slow down the reaction rate, providing better control over the reaction process.
- Economic considerations:Using an excess of a reactant can be more economical if that reactant is inexpensive or readily available.
Applications of Limiting and Excess Reactants: Limiting And Excess Reactants Pogil
Limiting and excess reactants find applications in various fields, including chemistry, industry, and everyday life. Understanding their roles is crucial for optimizing reactions and achieving desired outcomes.
In chemical reactions, limiting reactants determine the maximum amount of product that can be formed. By limiting the availability of one reactant, it ensures that the other reactants are present in excess, preventing wastage and ensuring efficient utilization of resources.
Industrial Applications, Limiting and excess reactants pogil
- Pharmaceutical Industry:Limiting reactants are used to control the production of specific drug molecules, ensuring precise dosages and minimizing side reactions.
- Chemical Manufacturing:Excess reactants are employed to drive reactions towards completion, maximizing product yield and minimizing the need for multiple reaction steps.
Environmental Applications
- Water Treatment:Limiting reactants are used to control the addition of chemicals, such as chlorine, to ensure effective disinfection without creating harmful byproducts.
- Pollution Control:Excess reactants can be used to neutralize pollutants in wastewater or exhaust gases, reducing their environmental impact.
Everyday Applications
- Cooking:Limiting reactants, such as spices or herbs, are used to control the flavor and intensity of dishes.
- Photography:Excess reactants are used in developing solutions to ensure complete conversion of unexposed silver halide crystals to metallic silver, resulting in clear and sharp images.
The choice between limiting and excess reactants depends on the specific application. Limiting reactants provide precise control over product formation, while excess reactants ensure high yields and minimize the need for additional steps. Understanding the advantages and disadvantages of each approach is essential for optimizing reactions and achieving desired outcomes.
Experimental Determination of Limiting Reactants
Determining the limiting reactant in a chemical reaction is crucial for predicting the maximum amount of product that can be formed. This section presents an experimental approach to identify the limiting reactant.
Procedures and Techniques
- Reactant Preparation: Prepare two solutions of reactants with known concentrations and volumes.
- Reaction Setup: Mix the reactants in a reaction vessel and allow the reaction to proceed.
- Reaction Monitoring: Monitor the reaction progress over time using techniques such as titration, spectrophotometry, or gas chromatography.
- Data Analysis: Plot the concentration or amount of reactants and products against time. Determine the point at which one reactant is completely consumed while the other reactant remains.
The reactant that is completely consumed first is the limiting reactant, as it limits the amount of product that can be formed. This experimental approach provides a quantitative method to determine the limiting reactant in a chemical reaction.
FAQ Overview
What is the significance of identifying limiting and excess reactants?
Identifying limiting and excess reactants is crucial for predicting the maximum yield of a reaction and optimizing resource allocation. It allows chemists to determine the exact amount of each reactant required to achieve the desired outcome, minimizing waste and maximizing efficiency.
How can stoichiometry help determine the limiting reactant?
Stoichiometry provides the mole ratios of reactants and products involved in a chemical reaction. By comparing these ratios to the initial amounts of reactants, we can identify the reactant that is present in the smallest relative amount, which will be the limiting reactant.
What are the implications of having an excess reactant?
Having an excess reactant ensures that the reaction can proceed to completion, even if other reactants are limiting. However, it can also lead to unreacted excess reactant remaining in the final product, which may need to be separated or removed.
