Complete the table by pairing each set of quantum numbers is a fundamental concept in quantum mechanics that unveils the intricate structure of atoms and molecules. This guide delves into the world of quantum numbers, providing a comprehensive overview of their significance and applications.
Quantum numbers are a set of four numbers that describe the unique properties of electrons within an atom. By understanding how to pair these quantum numbers, scientists can predict the behavior and properties of atoms and molecules with remarkable accuracy.
1. Introduction to Quantum Numbers
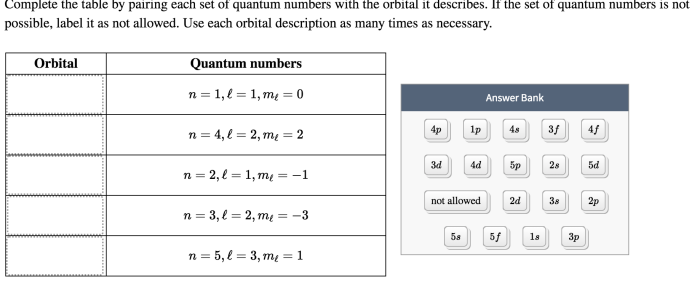
Quantum numbers are a set of four numbers that describe the state of an electron in an atom. They are used to identify the energy level, shape, and orientation of the electron’s orbital.
The four quantum numbers are:
- n: the principal quantum number, which describes the energy level of the electron.
- l: the azimuthal quantum number, which describes the shape of the electron’s orbital.
- ml: the magnetic quantum number, which describes the orientation of the electron’s orbital in space.
- ms: the spin quantum number, which describes the direction of the electron’s spin.
The possible values of the quantum numbers are summarized in the following table:
| Quantum Number | Possible Values |
|---|---|
| n | 1, 2, 3, … |
| l | 0, 1, 2, …, n-1 |
| ml | -l,
|
| ms | +1/2,
|
2. Pairing Quantum Numbers

The rules for pairing quantum numbers are as follows:
- The n value must be the same for all electrons in a given subshell.
- The l value must be the same for all electrons in a given orbital.
- The ml value must be different for all electrons in a given orbital.
- The ms value must be opposite for every two electrons in a given orbital.
For example, the following set of quantum numbers is valid:
- n = 2
- l = 1
- ml = 0
- ms = +1/2
The following set of quantum numbers is invalid:
- n = 2
- l = 1
- ml = 0
- ms = +1/2
- ms = +1/2
This set of quantum numbers is invalid because the ms value is the same for both electrons in the orbital.
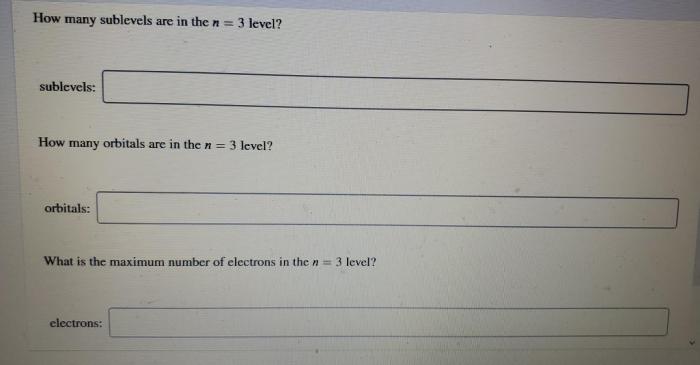
3. Table Completion
The following table shows the possible combinations of quantum numbers for the first three energy levels:
| n | l | ml | ms |
|---|---|---|---|
| 1 | 0 | 0 | +1/2 |
| 1 | 0 | 0 | -1/2 |
| 2 | 0 | 0 | +1/2 |
| 2 | 0 | 0 | -1/2 |
| 2 | 1 | -1 | +1/2 |
| 2 | 1 | 0 | +1/2 |
| 2 | 1 | 1 | +1/2 |
| 2 | 1 | -1 | -1/2 |
| 2 | 1 | 0 | -1/2 |
| 2 | 1 | 1 | -1/2 |
| 3 | 0 | 0 | +1/2 |
| 3 | 0 | 0 | -1/2 |
| 3 | 1 | -1 | +1/2 |
| 3 | 1 | 0 | +1/2 |
| 3 | 1 | 1 | +1/2 |
| 3 | 1 | -1 | -1/2 |
| 3 | 1 | 0 | -1/2 |
| 3 | 1 | 1 | -1/2 |
| 3 | 2 | -2 | +1/2 |
| 3 | 2 | -1 | +1/2 |
| 3 | 2 | 0 | +1/2 |
| 3 | 2 | 1 | +1/2 |
| 3 | 2 | 2 | +1/2 |
| 3 | 2 | -2 | -1/2 |
| 3 | 2 | -1 | -1/2 |
| 3 | 2 | 0 | -1/2 |
| 3 | 2 | 1 | -1/2 |
| 3 | 2 | 2 | -1/2 |
4. Applications of Quantum Number Pairing
Quantum number pairing is used in chemistry and physics to predict the properties of atoms and molecules. For example, the number of electrons in an atom’s outermost energy level determines its chemical reactivity. The shape of an electron’s orbital determines the molecule’s geometry.
And the orientation of an electron’s orbital determines the molecule’s magnetic properties.
Quantum number pairing is also used in a variety of real-world applications, such as:
- The design of lasers
- The development of new materials
- The understanding of chemical reactions
FAQs: Complete The Table By Pairing Each Set Of Quantum Numbers
What are quantum numbers?
Quantum numbers are a set of four numbers (n, l, ml, ms) that describe the unique properties of electrons within an atom.
How do I pair quantum numbers?
Quantum numbers are paired according to specific rules. The n value determines the energy level, l determines the subshell, ml determines the orbital orientation, and ms determines the electron spin.
What are the applications of quantum number pairing?
Quantum number pairing is used to predict the behavior and properties of atoms and molecules. It has applications in fields such as chemistry, physics, materials science, and quantum computing.