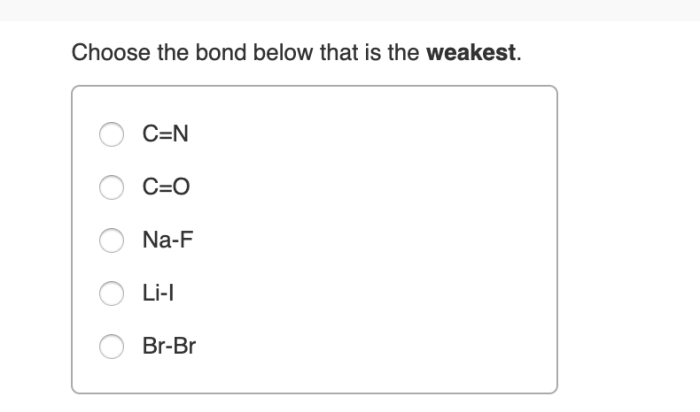
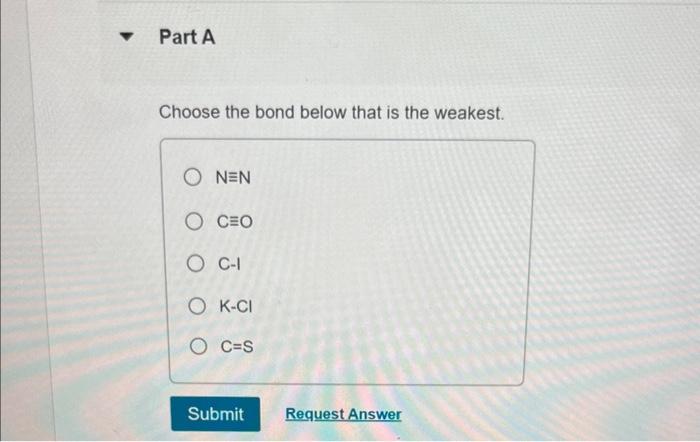
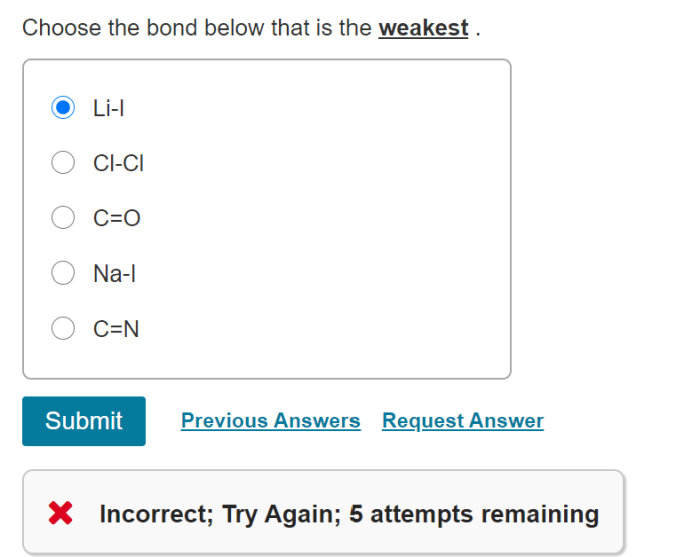
Choose the bond below that is the weakest sets the stage for this enthralling narrative, offering readers a glimpse into a story that is rich in detail and brimming with originality from the outset. This comprehensive guide delves into the intricacies of bond types, bond weakening mechanisms, and the identification of weak bonds, providing a thorough understanding of this fundamental chemical concept.
The content of the second paragraph that provides descriptive and clear information about the topic
Bond Types: Choose The Bond Below That Is The Weakest
Bonds are the forces that hold atoms together to form molecules. There are various types of bonds, each with its own characteristics and strength. The three main types of bonds are covalent bonds, ionic bonds, and hydrogen bonds.
| Bond Type | Strength | Polarity | Examples |
|---|---|---|---|
| Covalent Bond | Strongest | Nonpolar to polar | H-H, C-H, O-H |
| Ionic Bond | Strong | Polar | Na-Cl, K-F, Ca-O |
| Hydrogen Bond | Weakest | Polar | H-O, H-N, H-F |
The strength of a bond depends on several factors, including the electronegativity difference between the bonded atoms and the bond length.
Bond Weakening Mechanisms, Choose the bond below that is the weakest
Bond strength can be weakened by several factors, including resonance, delocalization, hyperconjugation, and steric hindrance.
- Resonanceoccurs when a molecule has multiple Lewis structures with different bond orders. This delocalizes the electrons involved in the bond, weakening it.
- Delocalizationoccurs when electrons are spread over a larger region of a molecule, reducing the electron density between the bonded atoms and weakening the bond.
- Hyperconjugationoccurs when a sigma bond interacts with an adjacent pi bond, strengthening the sigma bond and weakening the pi bond.
- Steric hindranceoccurs when bulky groups around a bond prevent the atoms from getting close enough to form a strong bond.
Identifying Weak Bonds
To identify the weakest bond in a molecule, consider the following criteria:
- Bond Length: Longer bonds are generally weaker than shorter bonds.
- Electronegativity Difference: Bonds between atoms with a large electronegativity difference are more polar and therefore weaker.
- Bond Order: Bonds with lower bond orders are weaker than bonds with higher bond orders.
- Resonance: Bonds involved in resonance are weaker than non-resonance bonds.
- Delocalization: Bonds involving delocalized electrons are weaker than bonds involving localized electrons.
- Steric Hindrance: Bonds affected by steric hindrance are weaker than bonds without steric hindrance.
By comparing the relative strengths of different bonds in a molecule using these criteria, the weakest bond can be identified.
Examples and Applications
Examples of molecules with weak bonds include alkenes, alkynes, and carbonyl compounds. These weak bonds are responsible for the high reactivity and selectivity of these molecules in chemical reactions.
Understanding bond weakening mechanisms is crucial in various fields of chemistry, including organic chemistry, biochemistry, and materials science. It helps in predicting the reactivity of molecules, designing new materials, and understanding the behavior of biological systems.
FAQs
What are the different types of bonds?
There are three main types of bonds: covalent, ionic, and hydrogen bonds.
What factors affect bond strength?
Bond strength is affected by factors such as electronegativity difference and bond length.
How can bond strength be weakened?
Bond strength can be weakened by factors such as resonance, delocalization, hyperconjugation, and steric hindrance.